Lung organoids unveil secret: How pathogens infect human lung tissue
How do pathogens invade the lungs? Using human lung microtissues, a team at the Biozentrum of the University of Basel has uncovered the strategy used by a dangerous pathogen. The bacterium targets specific lung cells and has developed a sophisticated strategy to break through the lungs’ line of defense.
10 June 2024 | Katrin Bühler
Earlier this year, the WHO published a list of twelve of the world’s most dangerous bacterial pathogens that are resistant to multiple antibiotics and pose a grave threat to human health. This list includes Pseudomonas aeruginosa, a much-feared nosocomial pathogen that causes severe and life-threatening pneumonia. This pathogen is especially threatening to immunocompromised patients and those on mechanical ventilation, with mortality rates up to 50 percent.
The lung barrier is penetrable
Pseudomonas aeruginosa has developed a broad range of strategies to invade the lungs and the body. Researchers led by Prof. Urs Jenal at the Biozentrum, University of Basel, have now gained novel insights into the infection process using lab-grown lung microtissues generated from human stem cells. In the scientific journal Nature Microbiology, they describe how Pseudomonas breaches the top layer of lung tissue and invades deeper areas. This study was conducted as part of the National Center of Competence in Research (NCCR) “AntiResist”.
Our lungs are lined by a thin layer of tightly packed cells that protects the deeper layers of lung tissue. The surface is covered with mucus, which traps particles such as microorganisms and is removed from the airways by specialized cells. This layer serves as an effective almost impenetrable barrier against invading pathogens. However, Pseudomonas bacteria have found a way to breach it. But how the pathogen crosses the tissue barrier has remained a mystery until now.
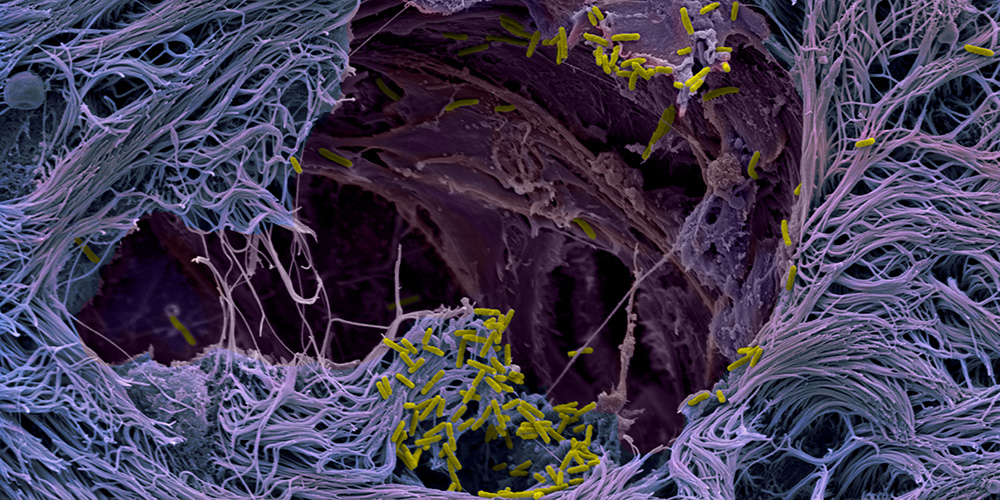
Lung organoids provide new insight into infections in humans
“We have grown human lung microtissues that realistically mimic the infection process inside a patient’s body,” explains Jenal. “These lung models enabled us to uncover the pathogen’s infection strategy. It uses the mucus-producing goblet cells as Trojan horses to invade and cross the barrier tissue. By targeting the goblet cells, which make up only a small part of the lung mucosa, the bacteria can breach the defense line and open the gate.”
With a large arsenal of virulence factors, known as secretion systems, the pathogen specifically attacks and invades the goblet cells, replicates inside the cells and ultimately kills them. The burst of the dead cells leads to ruptures in the tissue layer, making the protective barrier leaky. The pathogens exploit this weak spot: They rapidly colonize the rupture sites and spread into deeper tissue regions.
New sensor for monitoring bacteria
Using human lung organoids, the scientists have been able to elucidate the sophisticated infection strategies of Pseudomonas. However, it remains unclear how the pathogens adapt their behavior during the infection process. For example, they must first be mobile to spread over the tissue surface, then quickly adhere to lung cells upon contact, and later activate their virulence factors. It is known that the bacteria can rapidly change their behavior thanks to small signaling molecules. Until now, however, the technology to study these correlations was not available.
Jenal’s team has now developed a biosensor to measure and track a small signaling molecule called c-di-GMP in individual bacteria. The method was recently described in Nature Communications. "This is a technological breakthrough," says Jenal. "Now we can monitor in real time and with high resolution how this signaling molecule is regulated during infection and how it controls the pathogen’s virulence. We now have a detailed view on when and where individual bacterial cells activate certain programs to regulate their behavior. This method enables us to investigate lung infections in more detail."
Organ models mimic conditions in patients
"Thanks to the development of human lung organoids, we now have a much better understanding of how the pathogens behave in human tissue and presumably in patients," emphasizes Jenal. "This brings us a big step closer to the goal of NCCR AntiResist." Organoids of the human lung and other organs like the bladder allow the researchers to study the effects of antibiotics in tissue, for example, identifying where and how bacteria survive during treatment. Such organ models will be indispensable in the future for developing new and effective strategies to combat pathogens.
Original publications
A. Leoni Swart, Benoît-Joseph Laventie, Rosmarie Sütterlin, Tina Junne, Luisa Lauer, Pablo Manfredi, Sandro Jakonia, Xiao Yu, Evdoxia Karagkiozi, Rusudan Okujava and Urs Jenal.
Goblet cell invasion promotes breaching of respiratory epithelia by an opportunistic human pathogen.
Nature Microbiology (2024), doi: 10.1038/s41564-024-01718-6
Andreas Kaczmarczyk, Simon van Vliet, Roman Peter Jakob, Raphael Dias Teixeira, Inga Scheidat, Alberto Reinders, Alexander Klotz, Timm Maier, Urs Jenal.
A genetically-encoded biosensor to monitor dynamic changes of c-di-GMP with high temporal resolution.
Nature Communications (2024), doi: 10.1038/s41467-024-48295-0